NTE001 Study
A Phase I/II Clinical Trial of NRTX-1001 Nerve Cell Therapy in Drug-Resistant Unilateral Mesial Temporal Lobe Epilepsy
Information on NTE002, Click Here.


NRTX-1001 is an investigational drug (experimental drug). An investigational drug is one that the FDA and other health authorities have not yet approved to be safe and effective.
NRTX-1001 has undergone preclinical laboratory testing and received approval from the FDA to be tested in people.
NTE001 is the name of the clinical study designed to investigate the safety and efficacy of NRTX-1001 as a potential treatment for drug-resistant unilateral mesial temporal lobe epilepsy. Investigational study sites are currently screening adult patients in the United States for participation.

Purpose of the Study
This study is being conducted to gather information about the use of NRTX-1001 to treat seizures in people who have drug-resistant epilepsy. This is the first time NRTX-1001 is being tested in humans.
Epilepsy occurs when nerve cells send out abnormally ‘excited’ signals. A certain neurotransmitter, or messenger in the brain, called Gamma Aminobutyric Acid (GABA) can block or inhibit these excited signals. NRTX-1001 is made from human stem cells that have been developed into a non-dividing type of nerve cell called an interneuron, which releases GABA.
It is hoped that through implantation of NRTX-1001, the interneurons will form connections in the brain, and the release of GABA will quiet the nearby neurons and help limit the abnormally “excited” signals, resulting in fewer seizures.
What is NRTX-1001?
- NRTX-1001 is made from human stem cells that have been developed into interneurons (like the cells already in the brain).
- NRTX-1001 cells produce GABA, a neurotransmitter in the brain thought to inhibit seizure activity.
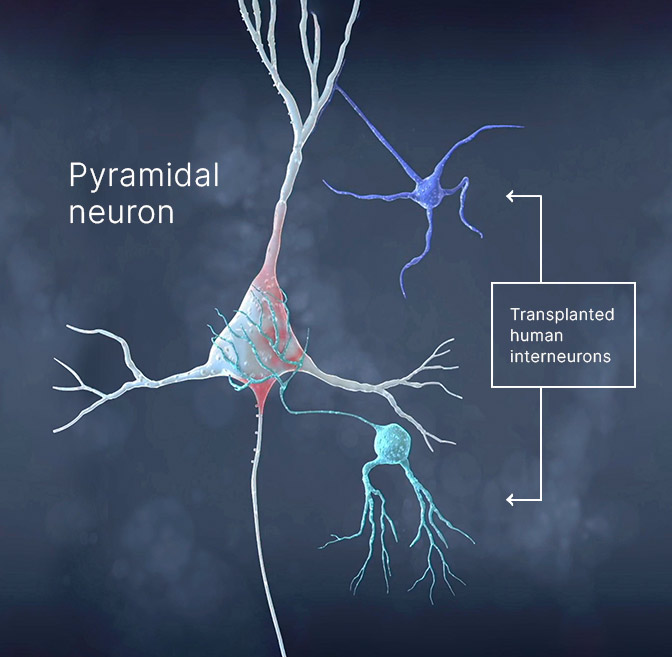
GABAergic interneuron cell therapy may restore missing inhibition and rebalance neural activity.
Qualifying for the Study
Are 18-75 years old
Have focal seizures, clinically defined as Temporal Lobe Epilepsy
Seizures haven’t been controlled by taking at least 2 seizure medications
Seizures confirmed by EEG and MRI to be coming from one location
Seizure frequency is at least 4 per 28-day period over the past 6 months
Considered to be a candidate for Temporal Lobectomy or Laser Ablation
No prior history of craniotomy
No prior exposure to gene or cell therapy treatments (of any kind)
Study Expenses
Neurona will reimburse the study subject and caregiver for reasonable travel expenses to confirm their eligibility and discuss participation with their doctor.
Once enrolled, they should not have to spend any of their own money to participate in the trial. All study costs are paid for by Neurona, including a service to help coordinate and pay for their travel arrangements.

Study Site Locations
Sites Currently Screening and Enrolling Patients
| State | City | Physician | Institution |
|---|---|---|---|
| AR | Little Rock | Sisira Yadala, MD | University of Arkansas |
| AZ | Phoenix | Jonathon Parker, MD, PhD | Mayo Clinic Arizona Epilepsy Center |
| AZ | Tucson | David Labiner, MD | Banner-University of Arizona Medical Center Tucson Comprehensive Epilepsy Program |
| CA | La Jolla | Jerry Shih, MD | University of California, San Diego Neurological Institute Epilepsy Center |
| CA | Los Angeles | Charles Y. Liu, MD, PhD | USC Comprehensive Epilepsy Center |
| CA | Los Angeles | John Stern, MD | University Of California Los Angeles Seizure Disorder Center |
| CA | Orange | Mona Sazgar, MD | UC Irvine Health |
| CA | Palo Alto | Kevin Graber, MD | Stanford Comprehensive Epilepsy Center |
| CA | Sacramento | Jack Lin, MD | University of California Davis Comprehensive Epilepsy Program |
| CA | San Francisco | Robert Knowlton, MD | University of California, San Francisco Seizure Disorders Surgery Program |
| CO | Aurora | Lesley Kaye, MD | University of Colorado Hospital Anschutz Outpatient Pavilion |
| FL | Miami | Jonathan Jagid, MD | University of Miami |
| IA | Iowa City | Mark Granner, MD | University of Iowa Hospitals and Clinics |
| IL | Chicago | Peter Warnke, MD | University of Chicago Comprehensive Epilepsy Center |
| IL | Chicago | Rebecca O’Dwyer, MD | Rush University Medical Center |
| LA | New Orleans | Fawad Khan, MD | The International Center for Epilepsy at Ochsner Medical Center |
| MA | Boston | Joshua Aronson, MD | Beth Israel Deaconess Medical Center |
| MI | Detroit | Maysaa Basha, MD | Wayne State University/Detroit Medical Center Comprehensive Epilepsy Program |
| NC | Charlotte | Rajdeep Singh, MD | Atrium Health |
| NC | Durham | Derek Southwell, MD, Ph.D. | Duke University |
| NC | Winston-Salem | Gautam Popli, MD | Wake Forest Baptist Health Comprehensive Epilepsy Center |
| NE | Omaha | Olga Taraschenko, MD, PhD | University of Nebraska |
| NY | New York | Orrin Devinsky, MD | NYU Langone Comprehensive Epilepsy Center |
| NY | Syracuse | Robert Beach, MD, Ph.D. | SUNY Upstate Medical University |
| OR | Portland | David Spencer, MD | Oregon Health & Sciences University |
| PA | Philadelphia | Michael Sperling, MD | Comprehensive Epilepsy Center at Thomas Jefferson University |
| TX | Houston | Nitin Tandon, MD | UT Health Houston Neurosciences |
| UT | Salt Lake City | Amir Arain, MD | University of Utah Clinical Neurosciences Center |
| WI | Milwaukee | Sean Lew, MD | Medical College of Wisconsin |
Resources
Disease awareness, patient advocacy, diversity in clinical trials
Commitment to Diversity and Inclusion in the NTE001 Study
Neurona is committed to diversity, equity, and inclusion of all races, ethnicities, and genders in the NTE001 clinical trial. The diversity of our clinical trial participants will best inform safety and efficacy of our cells in all populations. If you choose to participate in this trial, you will represent the safety and efficacy of our study for your demographic and community.
Contact Us
For more information about our clinical trials, contact us at clinicaltrialinfo@neuronatx.com.